We are the official independent global retrieval centre for the analysis of the recalled Stryker Rejuvenate and ABG II modular neck hips
It is speculated that increasing modularity in hips may increase the risk of failure due to corrosion and material loss at the head-neck and neck-stem junction. Understanding the mechanisms of material loss at the modular neck junction is a key area of research.
In July 2014 we signed a contract with Stryker to provide independent retrieval analysis of failed modular-neck hip implants. The project is in collaboration with Young-Min Kwon (MGH, USA). We are collecting implants together with clinical, imaging and laboratory data to determine a cause of failure. We retain the right to publish all material. Our objectives are as follows:
To gain further understanding of the mechanism of failure of modular neck hip prostheses
To risk stratify patients with ABGII and Rejuvenate modular neck stems
Development of early warning signs and clinical red flags for modular neck hips
Evidence to assist decision making for: who, when and how to revise.
Development of standardised protocols to minimise duplication/repetition
The findings of these studies will provide critical insights into the mechanism/s involved in failure of modular-neck femoral stems, thereby providing guidance to clinicians and research framework for further efforts to obtain evidence-based knowledge that would be pivotal for accurate and early detection of failure in pa- tients with modular-neck THA and optimise clinical management of these patients.


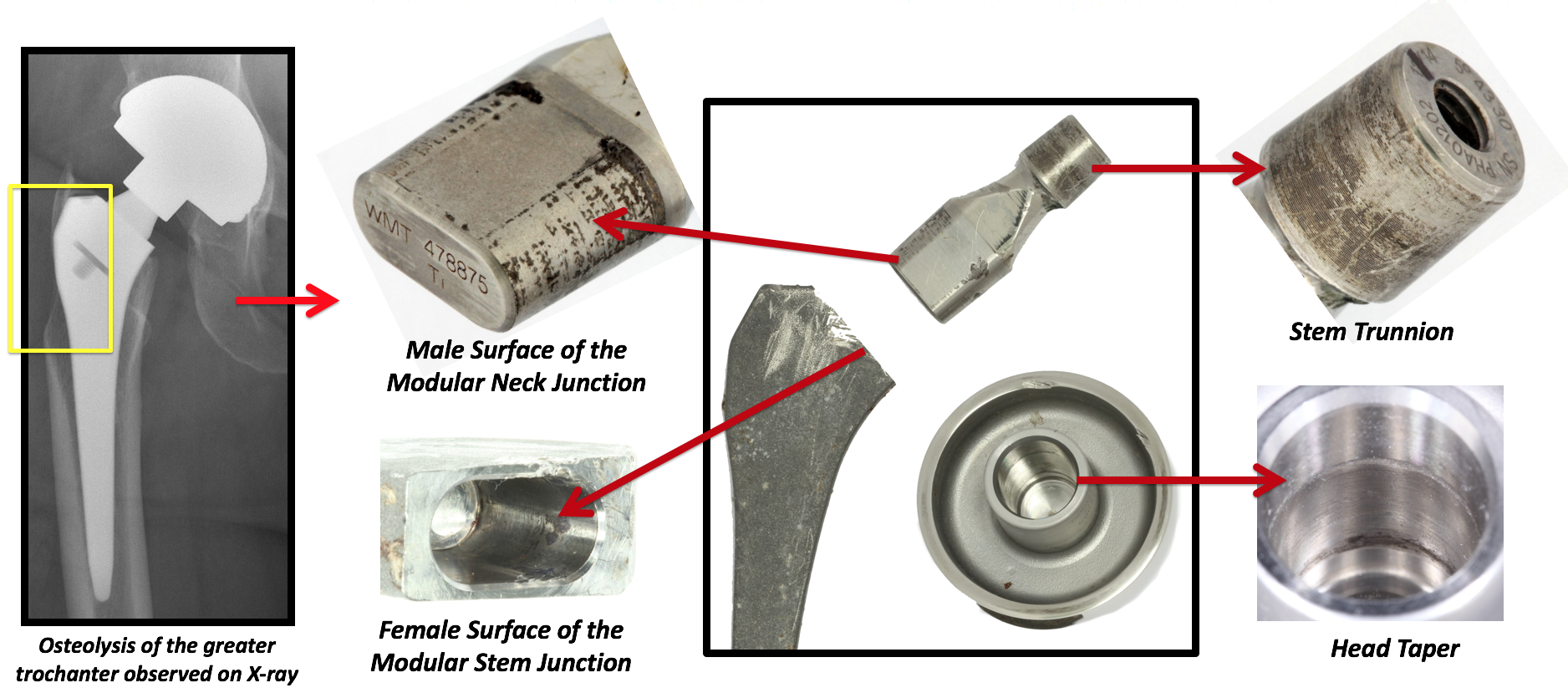





We will identify the surgical, implant and patient factors influencing failure; our centre has modular neck hips from different manufacturers and designs to use as comparison groups in our studies.


